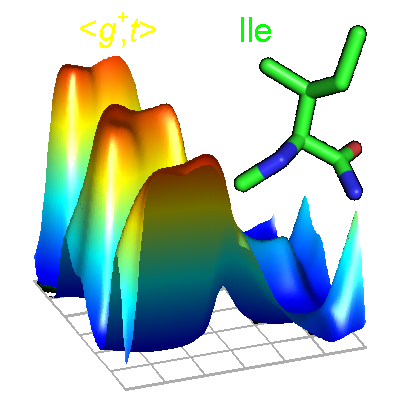
Fatal error: Uncaught Error: Call to undefined function mysql_connect() in /var/www/html/retro/bbdep2010/Main.php:18 Stack trace: #0 {main} thrown in /var/www/html/retro/bbdep2010/Main.php on line 18
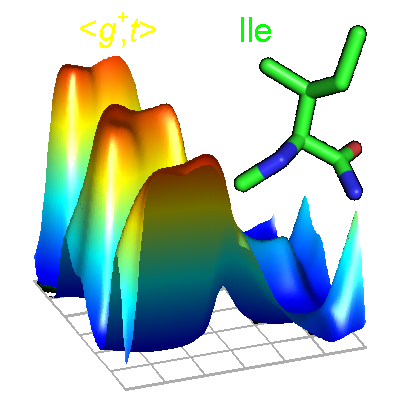
|
Fatal error: Uncaught Error: Call to undefined function mysql_connect() in /var/www/html/retro/bbdep2010/Main.php:18 Stack trace: #0 {main} thrown in /var/www/html/retro/bbdep2010/Main.php on line 18 |